
Pharmaceutical & Life Sciences


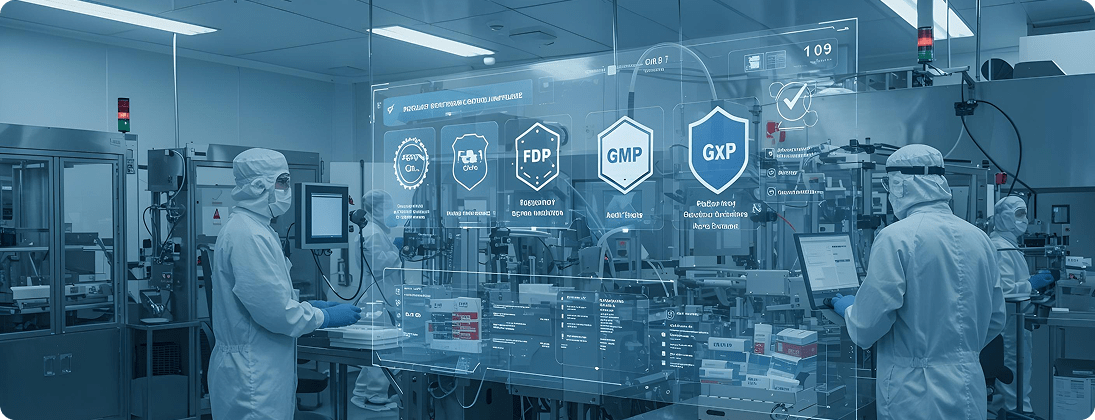
Unique Requirements and Challenges
Strict adherence to FDA 21 CFR Part 11, EMA Annex 11, GxP, and GMP is required. SAP standard often lacks fully validated processes.
Implement validated SAP environments with audit trails, electronic signatures, and GxP-compliant workflows. Provide CSV (Computer System Validation) and SOP alignment.
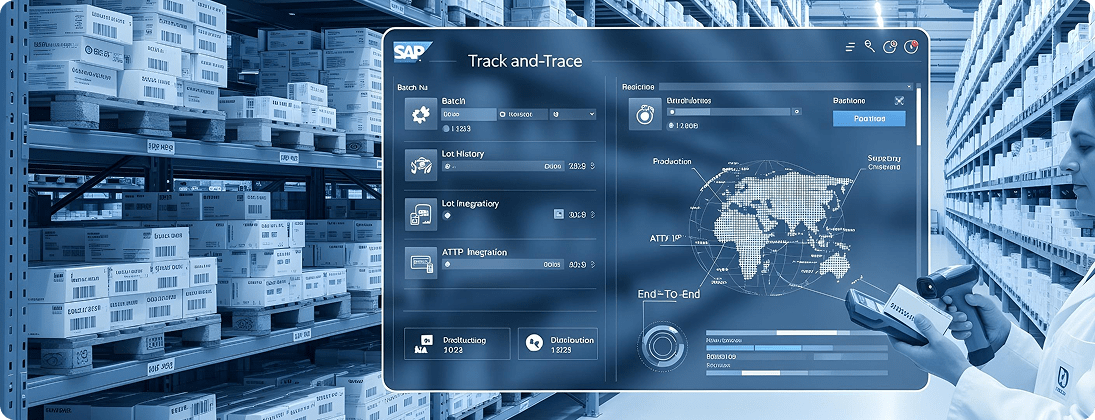
Unique Requirements and Challenges
Pharma requires end-to-end traceability of raw materials, intermediates, and finished drugs. Gaps can lead to recalls or compliance violations.
Configure SAP batch management with serialization and track-and-trace. Integrate SAP ATTP (Advanced Track and Trace for Pharmaceuticals) for end-to-end visibility.
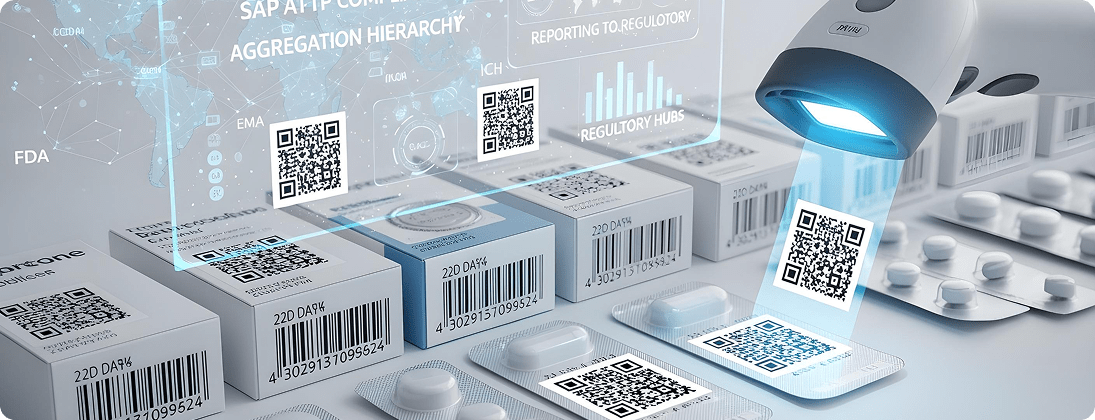
Unique Requirements and Challenges
Global regulations (DSCSA in US, FMD in EU, ANVISA in Brazil, etc.) mandate serialization to fight counterfeiting.
Deploy SAP ATTP and ICH-compliant solutions to manage serialization, aggregation, and reporting to regulatory hubs.
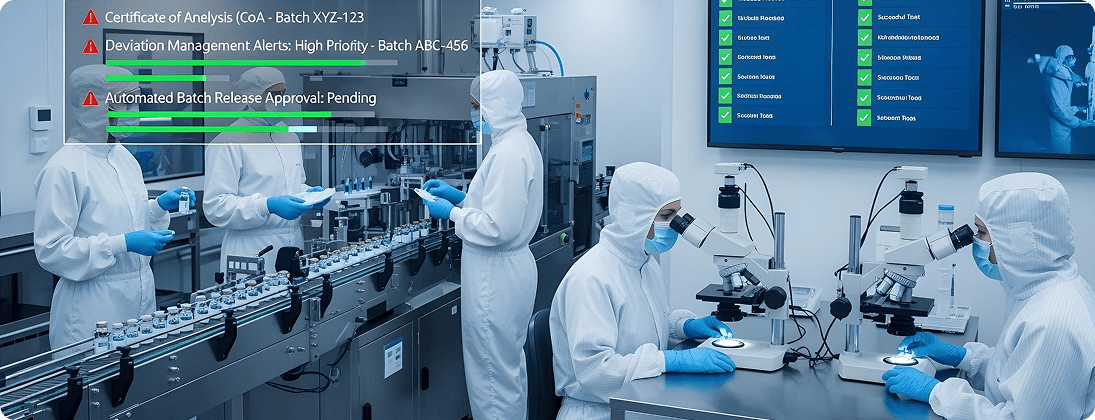
Unique Requirements and Challenges
In-process quality checks, batch release, and deviation management are often manual or disconnected from ERP.
Implement SAP QM integrated with production and procurement. Automate COA, deviation workflows, and batch release processes.
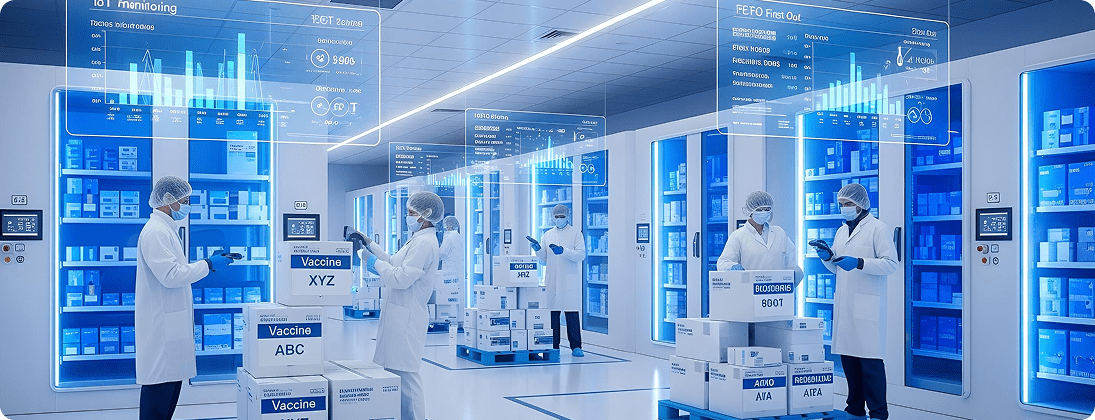
Unique Requirements and Challenges
Pharma products require strict temperature monitoring during storage and transport.
Configure SAP EWM with temperature zones and IoT integrations. Enable FEFO and real-time cold chain monitoring via barcode/RFID.

Unique Requirements and Challenges
Complex formulas, potency variations, and scaling are difficult to manage in SAP standard PP.
Optimize PP-PI recipes and production versions. Implement SAP PP-PI extensions for formula management with active ingredient calculations.

Unique Requirements and Challenges
Trial material distribution must be tracked globally with compliance.
Configure SAP for clinical supply chain visibility. Integrate with trial management systems for demand planning, labeling, and returns.
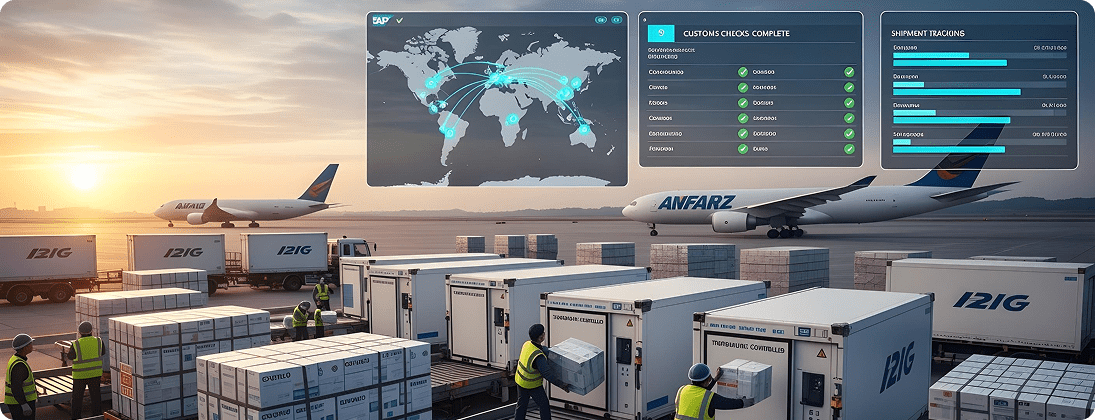
Unique Requirements and Challenges
Highly regulated international shipments face delays due to poor SAP–logistics integration.
Enable SAP GTS (Global Trade Services) for export/import compliance. Integrate logistics partners for seamless global distribution.
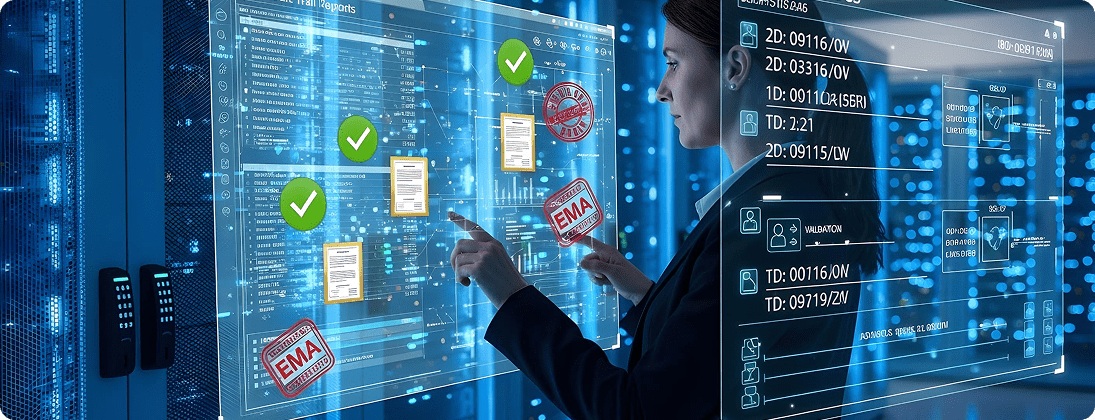
Unique Requirements and Challenges
Pharma requires validated data systems with audit-ready logs.
Ensure data integrity with SAP audit trails, role-based access, and validation documentation. Provide periodic compliance reporting.
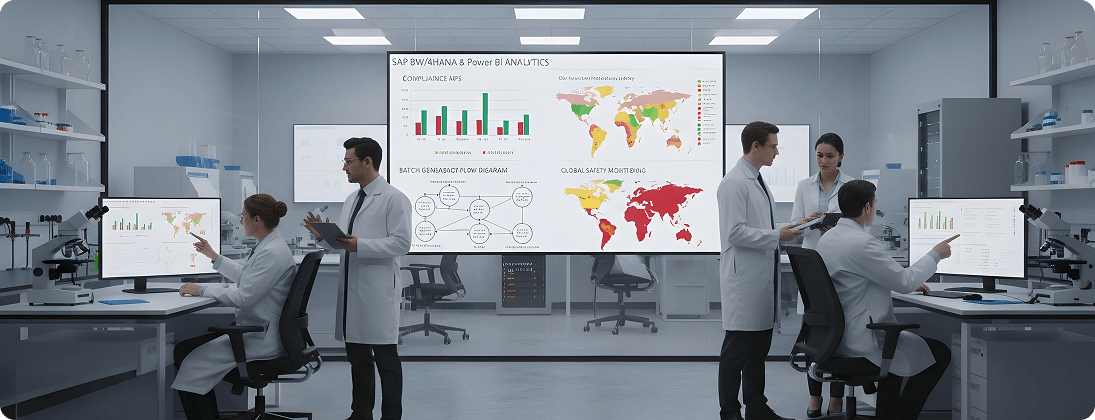
Unique Requirements and Challenges
Executives and regulators demand insights into quality, compliance, and product safety.
Build SAP BW/4HANA, Datasphere, or Power BI dashboards for compliance KPIs, batch genealogy, and global safety monitoring.